Dr. William Travis discusses the content of a poster session w/ Japanese surgeons. #WCLC2016 #LCSM #TogetherAgainstLungCancer
It has been a very busy week as I found the IASLC WCLC 2016 Lung Conference in Vienna so interesting.
I knew Merck were going to Publish My Trial Results MK3475-28 so I watched through each day and Tweeted all the Info I could to also share on my mesothelioma One Stop group on Face Book.
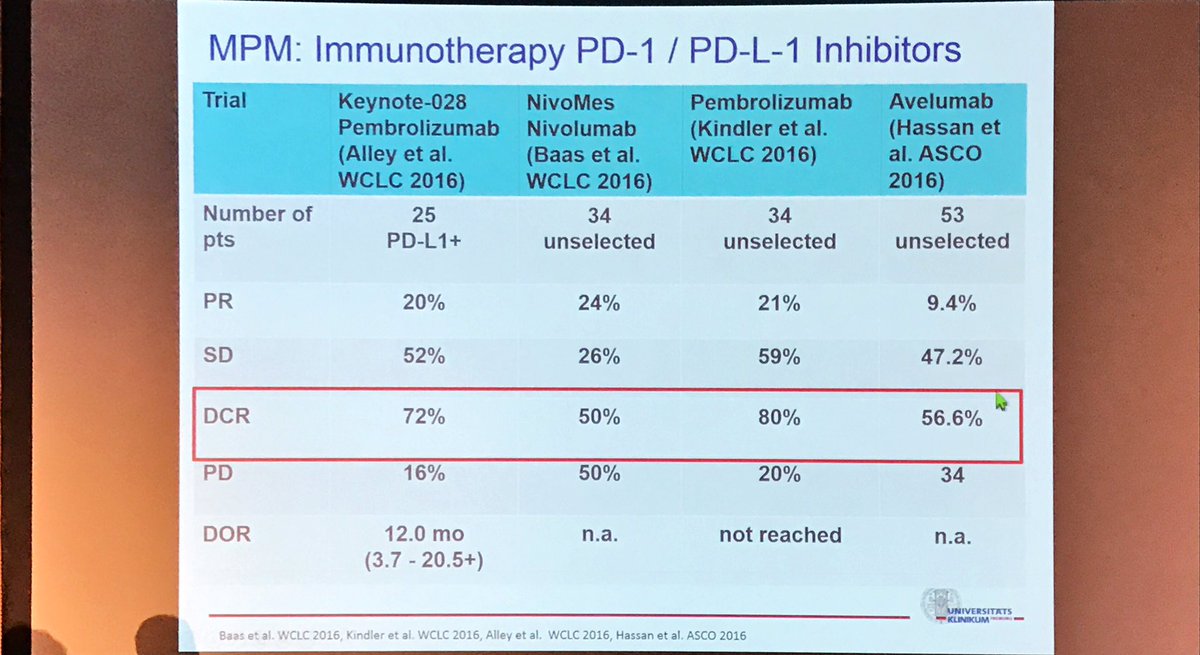
My Own Doctor Shah came up trumps for me with the picture that told a thousand words.
Immunotherapy cause a huge interest all week and the room was packed out with many even standing out side.
Merck had released this at the same time http://www.mercknewsroom.com/news-release/oncology-newsroom/updated-keytruda-pembrolizumab-data-small-cell-lung-cancer-and-mesoth
I was so proud I had taken part but a little perplexed as to why my result of C/R wasnt there. I’m asking the question everywhere as I don’t understand. Wasnt I finished in time ???
Press Conferences are at this link https://www.iaslc.org/news
Abstract Library is NOW OPEN
Access to abstracts is free of charge and can be viewed through the IASLC Virtual Library. Except for a select few presentations under embargo until their scheduled session time, all abstracts are now available
http://library.iaslc.org/virtual-library-search?product_id=6
As if this wasnt enough we have the Audit of 2014 from Mesothelioma UK
Overview of the results The audit collected data on 2,179 patients who were diagnosed with MPM in England in 2014, with a median of 13 cases per year for secondary care hospital trusts. This is the first national cancer audit to use Cancer Outcomes and Services Data (COSD) and cancer registry data directly to identify patients, which has enabled all cases of pleural MPM diagnosed in 2014 to be included in the audit.
The cancer registry data was supplemented with some data ssubmitted using the bespoke lung cancer dataset known as LUCADA. In view of the fact that a minority of hospital trusts submitted data solely via COSD and are thus not directly comparable, this 1-year interim report summarises results at national and strategic clinical network (SCN) level only.
Recording of key audit data is good, but variation exists in the data completeness of stage, performance status, multidisciplinary team (MDT) discussion and access to lung cancer nurse specialists (LCNS) across networks.
Although the overall pathological confirmation (following analysis of a tissue or fluid sample) of MPM is excellent (100% of cases), nearly half of MPM patients still receive an unspecified MPM diagnosis with no pathological subtyping. It is important that hospital trusts seek to improve this, since pathological subtype influences prognosis, and may affect eligibility or stratification for entry into clinical trials and response to systemic treatment.
In general, anti-cancer treatment and use of palliative chemotherapy has increased since the previous audit with 36.5% of all patients receiving it compared with 34% in the first report. In particular, for patients with good general health (performance status 0–1), chemotherapy delivery has increased to 53.5% cases compared with 41% previously. However, there is marked network variation ranging from 42.2% to 77.4%, which should be addressed.
Use of radiotherapy for MPM appears to have reduced since the last audit and was received by 16.5% of patients compared with 29% in the 2014 report.
Although the use of radical surgical treatment is extremely low in England, debulking surgical procedures (surgical removal of as much of a tumour as possible) in general do appear to have increased since the previous audit from 2.3% to 5.2%.
https://t.co/1Ovo0Iohab








Leave A Comment